Best practices and unique perspectives on root causes and developing corrective action plans. Having a good and reliable corrective and preventive action procedure is critical to the success of your company.

Corrective Action Plan Template Excel Templates
Corrective actions can be thought of as improvements to an organization to eliminate undesirable effects.

Why are corrective action plans important. Corrective and preventive action (capa) plans. Why a corrective action plan is important think of a corrective action plan as a record of your actions. By tammie van buren, manager, compliance.
It is essentially a plan to improve performance and/or reduce risk. A corrective action plan is an organizational document that describes exactly how a specific problematic situation will be changed to better meet the goals of a. But not asked for preventive action.
The cap gives you a reference point to look back on when there are questions about implementation and when it comes time to evaluate whether or not the changes were successful. Why is a good corrective and preventive action procedure important? Same mentioned in clause no.10.2 nonconformity and corrective action in iso 9001:2015 standard.
Corrective action is an aspect of quality management that aims to rectify a task, process, product, or even a person’s behavior when any of these factors produce errors or have deviated from an intended plan. Simply put, a correction is an immediate action taken to fix an issue identified during an audit or while monitoring and corrective action works to resolve the root cause of the issue. Whenever possible, the corrective action process should be a positive collaboration between the supervisor and employee to achieve necessary improvement rather than a punitive action against the employee.
The following are illustrative examples. Importance of corrective and preventive action (capa) corrective and preventive action is useful to eliminate the problem occurred during the manufacturing process. The corrective action plan is about addressing the root cause of the problem, not simply correcting the symptom that has been found.
A corrective action plan is a documentation used in quality management that outlines a set of steps for addressing issues and gaps in business operations and processes that could negatively impact the business. Corrective actions are those taken to resolve a problem and preventive actions are those actions that keep the problem from recurring. A cap is important because:
At its core, a corrective action plan is most needed when problems arise that pose a real threat to an organization’s quality management system or health and safety management. It’s a way of documenting issues and agreeing a shared approach to addressing them in order to bring a project back into a situation that feels in control. As you know the new iso 9001:2015 standard asked about correction, corrective action, and risk & its mitigation plan.
In the event of an audit A cap is important because: A corrective action plan is an organizational document that describes exactly how a specific problematic situation will be changed to better meet the goals of a.
Some examples of when a corrective action plan could be of assistance include incidents when nonconformity or poor quality is discovered as the result of an audit or customer complaint. And for retained documented information, iso 9001 asked mandatory requirement as evidence. It describes the approach for resolving an issue that interferes with reaching company goals.
Why caps rely so heavily on root causes, and how to help ensure, as compliance, that you get it right the first time through. The objective of corrective action is to correct and resolve employee performance problems in order to retain the employee as a productive staff member. Measuring and monitoring results to ensure the corrective action is truly corrective
What is a corrective action plan? The fda indicates that corrective and preventive actions (capas) are absolutely necessary to resolve problems and noncompliance in clinical investigations. The quality manager can try to respond to the corrective actions, but discover that not all of the information is available, or has the authority to make the needed changes.
Any time you have any nonconformity, you will be taking steps to correct the nonconformity, but what you correct is the difference between a simple correction and a corrective action.

How Do I Create An Effective Corrective Action Plan Nqa
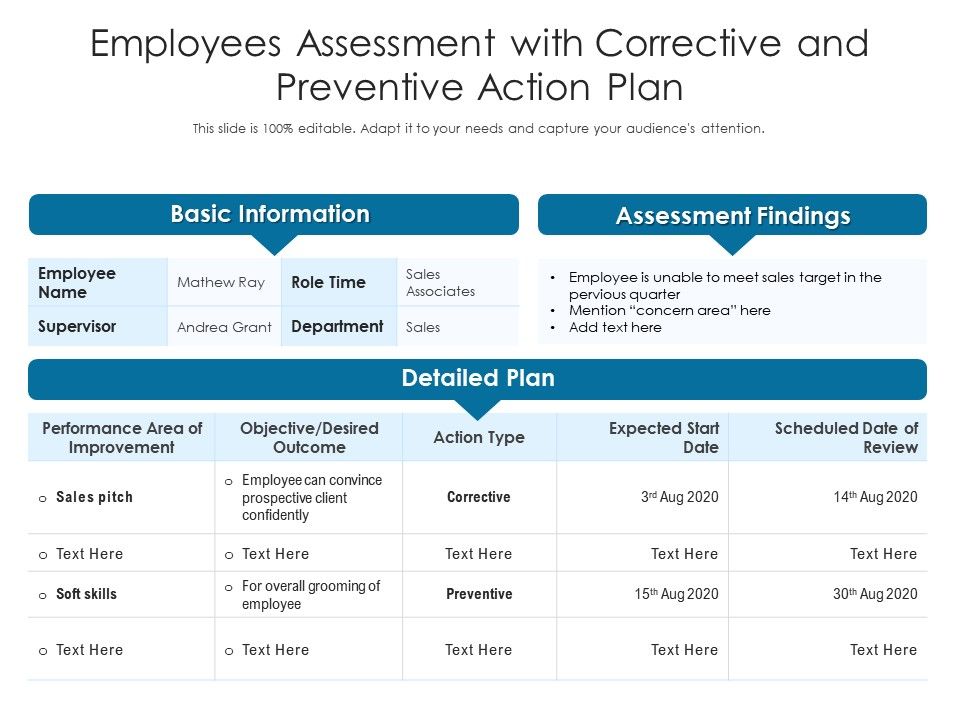
Employees Assessment With Corrective And Preventive Action Plan Presentation Graphics Presentation Powerpoint Example Slide Templates

Correction Corrective Action And Preventive Action - We Ask And You Answer The Best Answer Wins - Benchmark Six Sigma Forum
2

Corrective Action Plan Template - Pdfsimpli
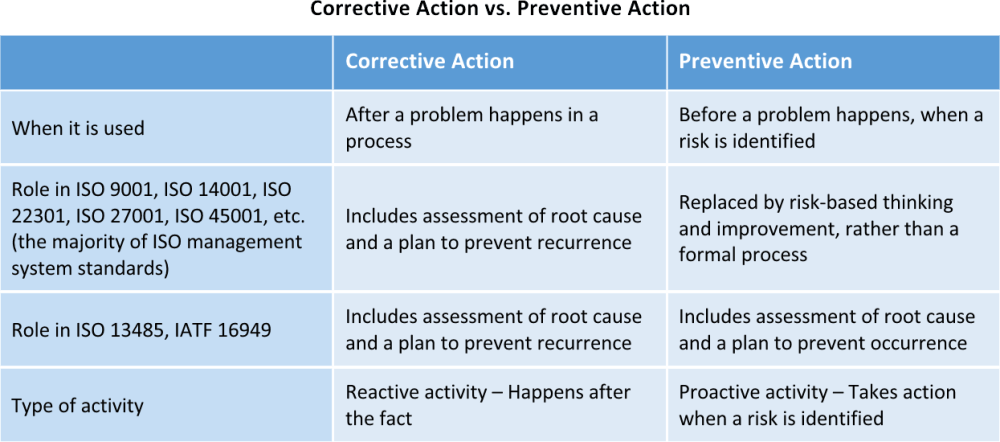
Corrective Action Vs Preventive Action A Complete Guide
Examples Of Fsma Corrective Action Plans In Practice - Corvium

Corrective Action Plan Report And Examples Safetyculture

Corrective Action Plan Report And Examples Safetyculture
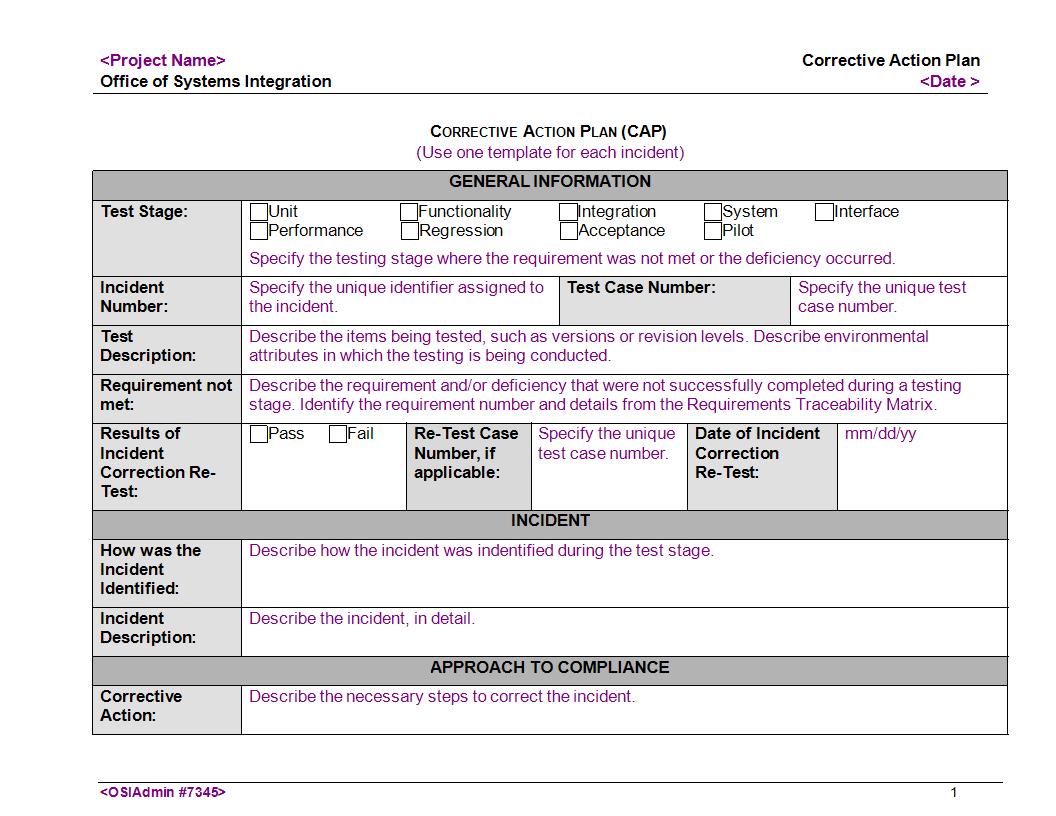
11 Corrective Action Plan Examples In Word Examples
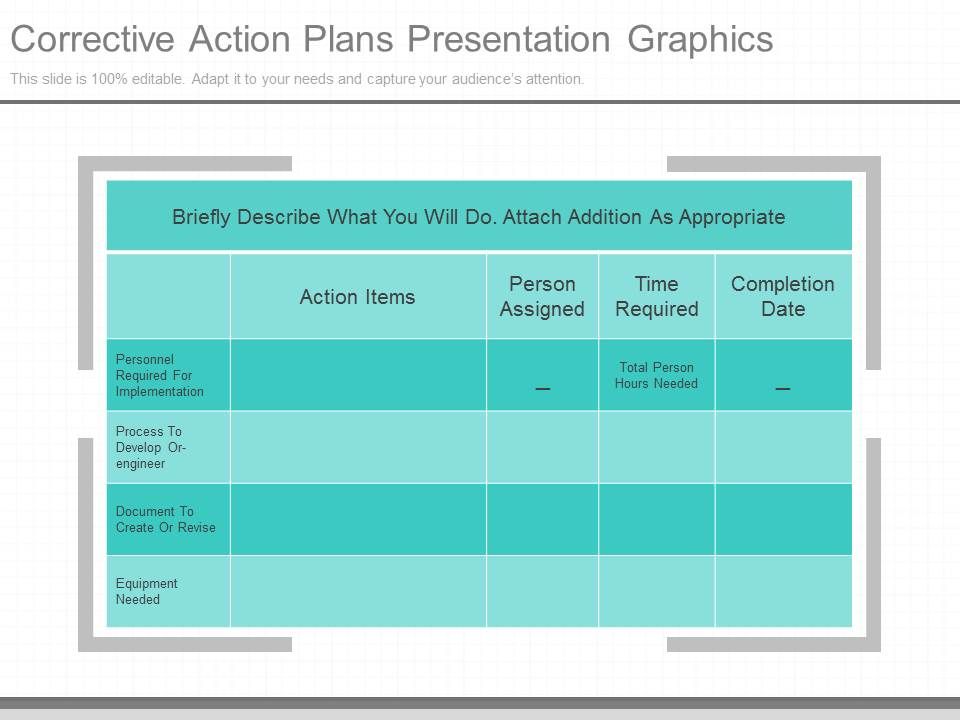
Original Corrective Action Plans Presentation Graphics Powerpoint Templates Download Ppt Background Template Graphics Presentation
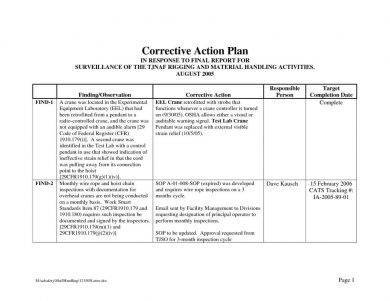
11 Corrective Action Plan Examples - Word Pdf Examples

How Do I Create An Effective Corrective Action Plan Nqa
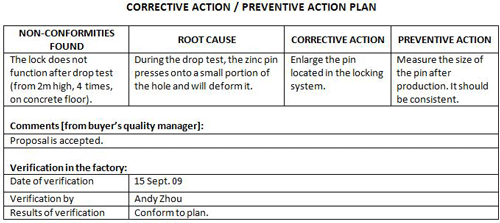
Use Corrective Actions Plans To Ensure Effective Repairing - Qualityinspectionorg

Corrective Action Plan What It Is And Implementation Tips - Sling

Corrective Action Plan Template Example For Teams Miro
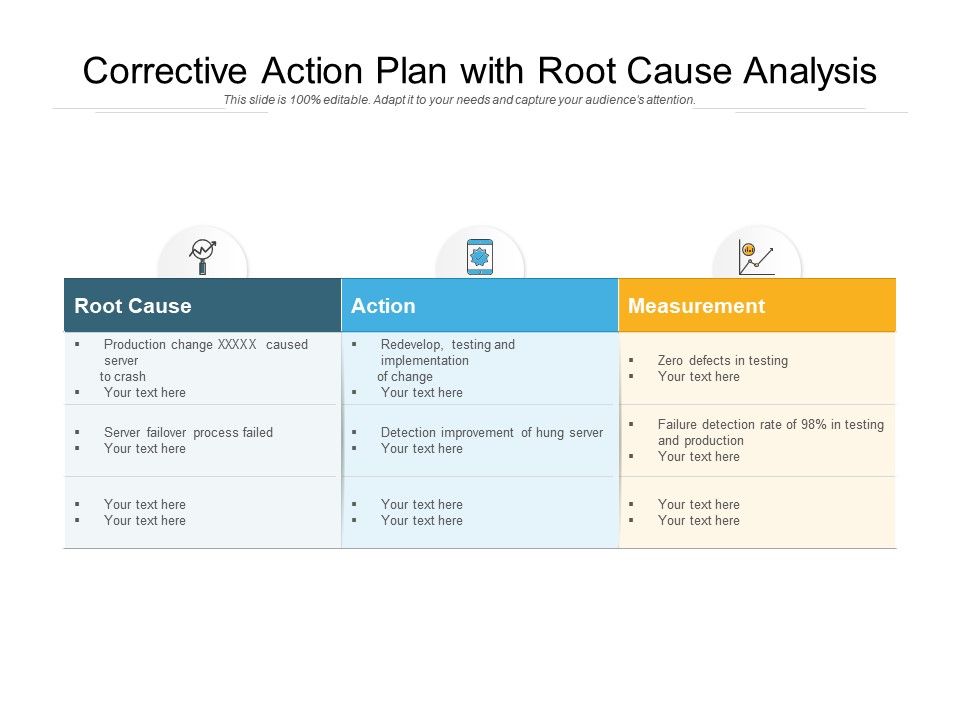
Corrective Action Plan With Root Cause Analysis Powerpoint Slides Diagrams Themes For Ppt Presentations Graphic Ideas

Employee Performance Management Corrective Action Plan Powerpoint Slides Diagrams Themes For Ppt Presentations Graphic Ideas

Employee Audit Corrective Action Plan Template Action Plan Template How To Plan Action Plan